截止目前,引用Bioss产品发表的文献共27040篇,总影响因子129798.43分,发表在Nature, Science, Cell以及Immunity等顶级期刊的文献共62篇,合作单位覆盖了清华、北大、复旦、华盛顿大学、麻省理工学院、东京大学以及纽约大学等国际知名研究机构上百所。
我们每月收集引用Bioss产品发表的文献。若您在当月已发表SCI文章,但未被我公司收集,请致电Bioss,我们将赠予现金鼓励,金额标准请参考“发文章 领奖金”活动页面。
近期收录2023年10月引用Bioss产品发表的文献共269篇(图一,绿色柱),文章影响因子(IF) 总和高达1698.2,其中,10分以上文献34篇(图二)。
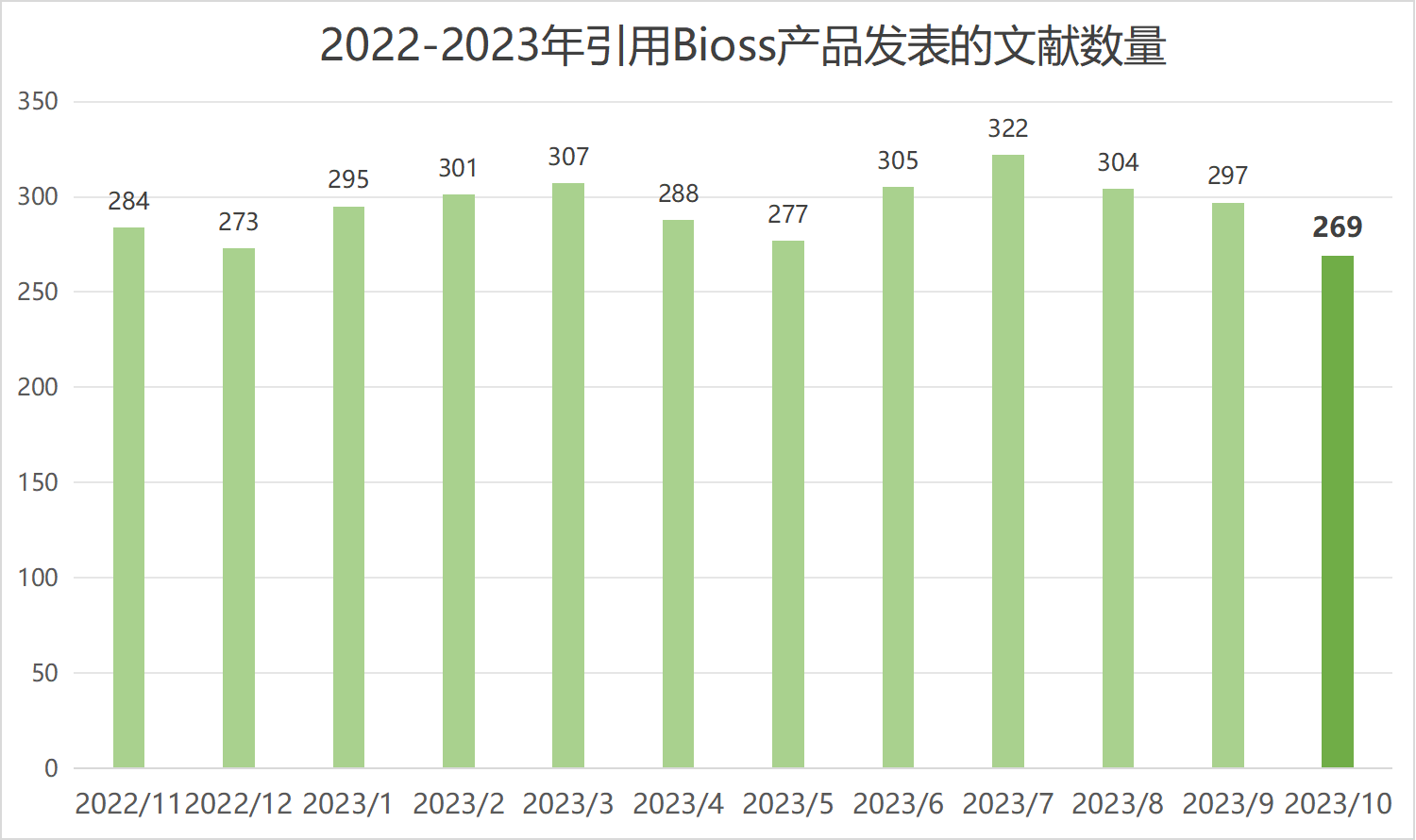
图一
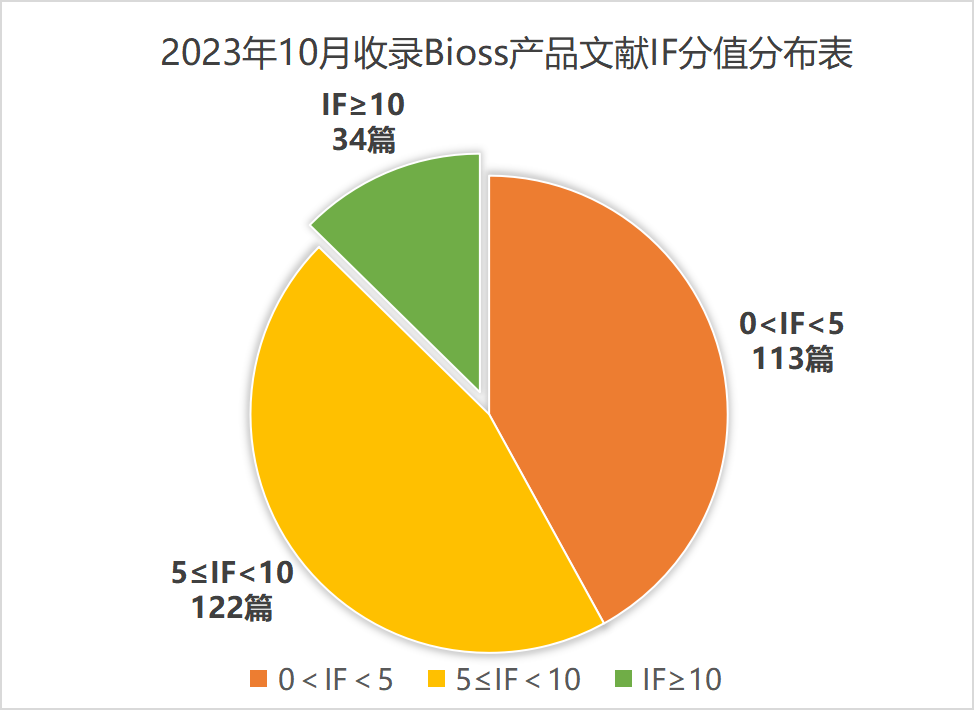
图二
本文主要分享引用Bioss产品发表文章至Nano Today/ Immunity / Cancer Cell等期刊的4篇IF>15的文献摘要,让我们一起欣赏吧。
文献引用产品:bs-10900R
GAPDH Rabbit pAb| WB
作者单位:中国科学院纳米材料生物医学效应与纳米安全重点实验室、南方医科大学
摘要:High-rate aerobic glycolysis and abnormal glutamine metabolism in tumor cells lead to their unlimited malignant proliferation and induce immune escape in the tumor microenvironment. In this study, the GLS1 inhibitor BPTES and the PDHC inhibitor CPI-613 were co-delivered by an ROS-sensitive GEM nano-prodrug (PD-G@BC), and a simultaneous inhibition of glycolysis and glutamine metabolism was achieved to deprive tumor cells of nutrient supply. In addition, this metabolism reprogramming effectively weakened the sources of glucose and glutamine in tumor cells, correspondingly thrived metabolism in antitumor immune cells, thereby increasing the intratumoral infiltration and functions of the immunogenic cells to alleviate the immunosuppressive responses. This multifunctional combination strategy will provide new insights into the design of synergistic cancer immunotherapy based on metabolic interventions.
ACS Nano [IF=17.1]

文献引用产品:
bsm-51215M; Hsp90 Mouse mAb | WB
bs-0126R; HSP70 Rabbit pAb | WB
作者单位:重庆大学
摘要:Low-temperature photothermal therapy (PTT) is a noninvasive method that harnesses the photothermal effect at low temperatures to selectively eliminate tumor cells, while safeguarding normal tissues, minimizing thermal damage, and enhancing treatment safety. First we evaluated the transcriptome of tumor cells at the gene level following low-temperature treatment and observed significant enrichment of genes involved in cell cycle and heat response-related signaling pathways. To address this challenge, we have developed an engineering multifunctional nanoplatform that offered an all-in-one strategy for efficient sensitization of low-temperature PTT. Specifically, we utilized MoS2 nanoparticles as the photothermal core to generate low temperature (40–48 °C). The nanoplatform was coated with DPA to load CPT-11 and Fe2+ and was further modified with PEG and iRGD to enhance tumor specificity (MoS2/Fe@CPT-11-PEG-iRGD). Laser- and acid-triggered release of CPT-11 can significantly increase intracellular H2O2 content, cooperate with Fe2+ ions to increase intracellular lipid ROS content, and activate ferroptosis. Furthermore, CPT-11 induced cell cycle arrest in the temperature-sensitive S-phase, and increased lipid ROS levels contributed to the degradation of HSPs protein expression. This synergistic approach could effectively induce tumor cell death by the sensitized low-temperature PTT and the combination of ferroptosis and chemotherapy. Our nanoplatform can also maximize tumor cell eradication and prolong the survival time of tumor-bearing mice in vivo. The multifunctional approach will provide more possibilities for clinical applications of low-temperature PTT and potential avenues for the development of multiple tumor treatments.
Nature Communications [IF=16.6]

文献引用产品:bs-1479R
CD80(B7-1) Rabbit pAb | WB
作者单位:沈阳药科大学五亚创新学院、新加坡国立大学杨潞龄医学院
CHEMICAL ENGINEERING JOURNAL [IF=15.1]
文献引用产品:S0286
1 % crystal violet solution
作者单位:南京大学医学院附属医院南京口腔医院
摘要:The reconstruction of alveolar bone defect is essential for periodontal regenerative therapy or subsequent prosthetic/implant therapy, particularly in individuals with systemic diseases, such as osteoporosis. ROS accumulation, coupled with aberrant macrophage polarization, is pivotal in the pathogenesis of osteoporosis and contributes to the disequilibrium between bone and the immune system. Consequently, therapeutic strategies targeting the immune microenvironment present a promising approach to the treatment of in situ alveolar bone regeneration in osteoporotic condition. Herein, we present a novel nano platform denoted as RSV@DTPF, designed to target macrophage and facilitate the controllable release of resveratrol in a ROS-responsive behavior. This approach aims to modulate the tampered immune microenvironment. The conjugated folate moiety selectively interacts with the folate receptors expressed on the macrophage surface, thereby augmenting cellular uptake. While the thioketal bond would break down when sensing a high level of intracellular ROS and release the encapsulated RSV. In vitro experiments suggested that designated nanoparticles could scavenge ROS effectively and restore the M1/M2 ratio in a lipopolysaccharide (LPS) -stimulated inflammation environment; The co-culture experiment provided evidence substantiating the adeptness of the modified immune milieu in fostering osteoblast differentiation, while concurrently impeding osteoclast maturation. Furthermore, through comprehensive imaging and histopathological evaluations, we elucidate the potential of RSV@DTPF in promoting osteogenesis within a periodontal defect model utilizing ovariectomized (OVX) rats. This in vivo study substantiates the advantageous impact of the nanoplatform on alveolar bone regeneration and its associated immunomodulatory effects. In summary, the RSV@DTPF nanoplatform exhibited the capacity to orchestrate immune microenvironmental shifts and enhance alveolar bone generation ability in osteoporosis and also could be expected to be applied in other biomedical fields associated with redox metabolism imbalance.